

It has an atomic mass of 14 amu and is radioactive. Carbon-13 has 7 neutrons: mass number of 13 - 6 protonsĬarbon-14 is a rare isotope of carbon, making up about 1 part per trillion of naturally occurring carbon. It has an atomic mass of 13 amu and is also stable.

Carbon-12 has 6 neutrons, which is calculated by taking the mass number of 12 and subtracting 6 protons.Ĭarbon-13 is a less abundant isotope of carbon, making up about 1.1% of naturally occurring carbon. It has an atomic mass of 12 amu and is stable. There are three naturally occurring isotopes of carbon: carbon-12, carbon-13, and carbon-14.Ĭarbon-12 is the most abundant isotope of carbon, making up about 98.9% of naturally occurring carbon.

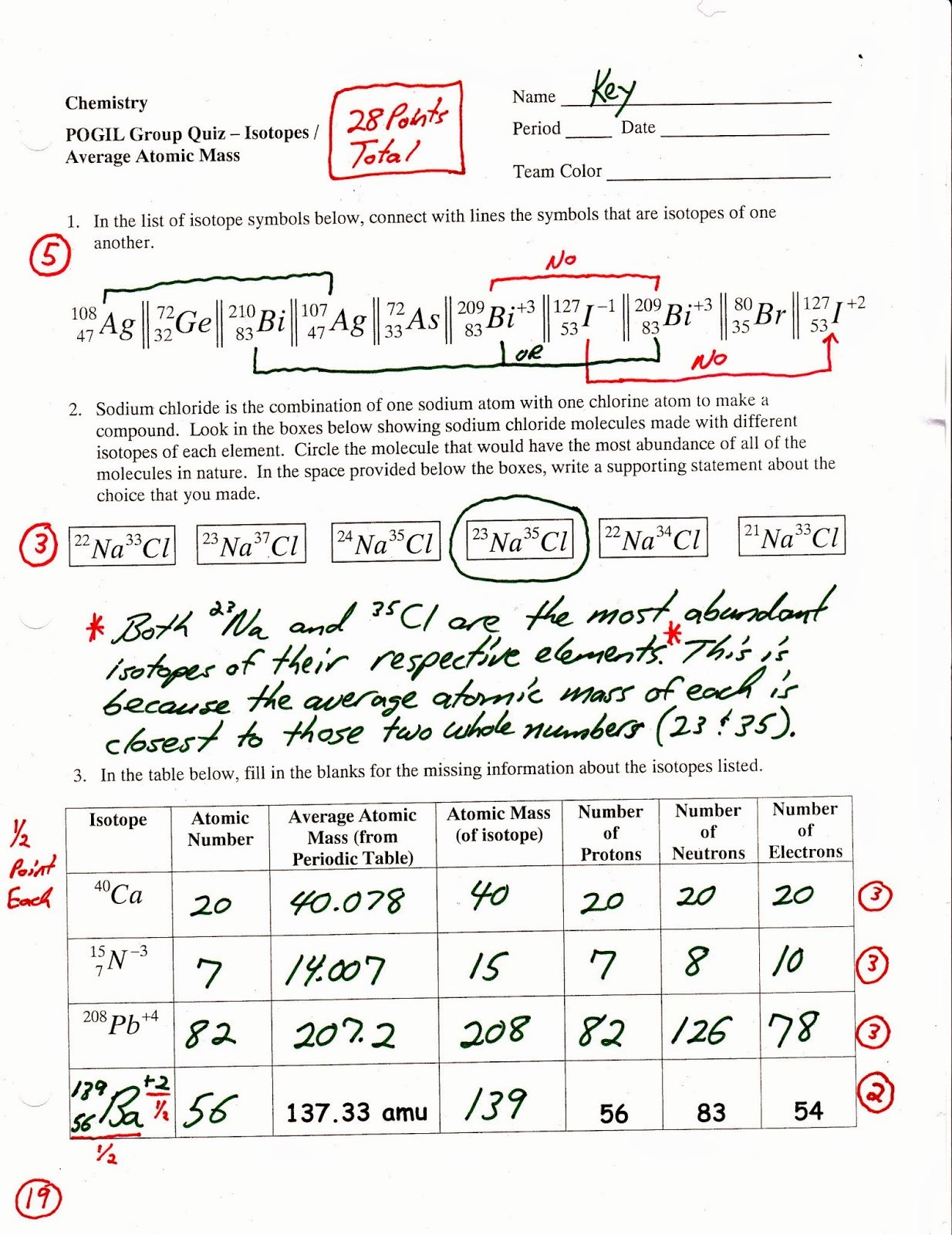
🤔 Carbon's 3 Naturally Occurring Isotopes Therefore, the average atomic mass represents all of the isotopes of an atom, and how often they occur naturally in the environment. This means that isotopes have the same atomic number (number of protons) but a different atomic mass (total number of protons and neutrons). They have the same number of protons and electrons, but a different number of neutrons. 🎥 Watch Jacob Jeffries discuss the parts of the atom and the experiments scientists use to study them. The average atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes of that element, based on their relative abundances. This is all because the atomic masses that you're given on the periodic table are actually the average atomic masses of these elements. Hydrogen has an atomic mass of 1.008, and iron (Fe) has an atomic mass of 55.85. If you take a look at other elements on the periodic table, you'll notice a similar trend. Instead of a nice "12" or "13" under Carbon, there's a really messy decimal of 12.01. Here is what we know so far about carbon based on the periodic table:Ĭarbon is represented by the element symbol "C."Ĭarbon's atomic number is 6, which means one atom of carbon has 6 protons in its nucleus and 6 electrons orbiting its nucleus.Ĭarbon's atomic mass looks to be 12.01, but how would this make sense? Since atomic mass = protons + neutrons, and we know we have 6 protons, that would give us 6.01 neutrons. Let's take a look at carbon on the periodic table that will be given to you during the AP exam: This will be our focus The atomic mass of elements represented on the periodic table will be our focus in this guide! The atomic mass is a representation of the number of protons in an atom of that element + the number of neutrons in an atom of that element. It is also the number of electrons in a neutral atom of that element, which we will discuss more later in this unit.Ītomic mass: The atomic mass of an element is found below the element symbol and is typically expressed in atomic mass units (amu). On the periodic table, you can find the following information for each element:Įlement symbol: Each element is represented by a unique symbol, typically consisting of one or two letters.Ītomic number: The atomic number of each element, found above the element symbol, represents the number of protons in the nucleus of the atom of that element. Understanding these three subatomic particles and how they contribute to the makeup of the periodic table is significant. When taking a look at the periodic table, you may wonder how scientists discovered all these numbers! As we discussed in the last section of this unit, an atom is made up of protons, neutrons, and electrons.


 0 kommentar(er)
0 kommentar(er)
